Synthesis of Distorted Nitrogen-Doped Nanographenes
We synthesized the distorted nitrogen-doped nanographenes which differ by a single C−C bond, and confirm that the cyclodehydrogenation is a stepwise ring-closing process. The priority of C−C bond formation has been proposed based on experimental investigations and theoretical calculations. Photophysical and electrochemical studies reveal the ring-fusing process affects the optoelectronic properties of nanographene.
Aggregation-Induced Emission Luminogens
This work provides a facile method for constructing Aggregation-Induced Emission (AIE)- and Mechanochromic (MC)-active luminogens, paving the way for further research on mechanochromism guided by a clearer understanding of the relationship between molecular packing and MCL properties.
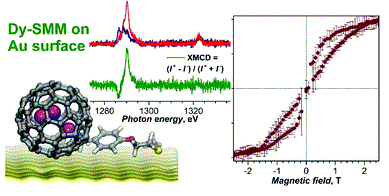
Functionalized Fullerenes in Self-Assembled Monolayers
Fullerene single molecule magnets (SMMs) DySc2N@C80 and Dy2ScN@C80 are functionalized via a 1,3-dipolar cycloaddition with surface-anchoring thioether groups. The SMM properties of Dy-fullerenes are substantially affected by the cycloaddition. Submonolayers of the physisorbed derivatives exhibit magnetic hysteresis on an Au(111) surface at 2 K as revealed by X-ray magnetic circular dichroism.